Hey Mama, “do you know how soap works?” You can face this question anyday from your school going kid, when she return back to home from her science classes. Are you ready to answer it to your curious kid?
When your kids read about saponification and the cleaning function of soap in their chemistry syllabus, it is difficult for them to observe the chemical reactions of soap. They obviously come to you with their queries. Now the question is how to demonstrate them that how soap works?
Today I am demonstrating “How soap cleans” with an amazing explosion of colors in milk. It is a very easy and funny science experiment for kids which you can do with general household items and can easily teach your kids how soap works.
Things required for experiment :
- Plate (glass dish is best) – 1
- Milk (to pour in dish)
- Food colors of your choice
- Liquid soap or shampoo
- Few droppers
How Soap Works : Video
Experiment Steps to Learn How Soap Works:
- Pour milk in a plate and let it settle.
- Add few drops of food colors in it at center with the help of droppers.
- Now fill liquid soap or shampoo in a dropper and drip a drop gently over food colors in milk and see the magic.
- Wow! You will instantly find a wonderful, amazing explosion of colors in milk!
Why It Happens:
Soap has a bipolar characteristic. It has one polar end and one non polar end. When soap is used for cleaning, it allows insoluble particles to become soluble in water and then be rinsed away.
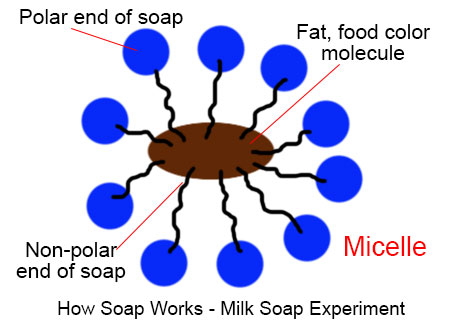
Here, milk has fat contents and water. When a drop of liquid soap or shampoo is added to the milk, the insoluble fat molecules (present in milk) become associated inside micelles, tiny spheres formed from soap molecules with polar hydrophilic (water-attracting) groups on the outside and encasing a lipophilic (fat-attracting) pocket, which shields the fat molecules from the water (and milk) making it soluble.
These fat molecules are so tiny that we can’t see those with naked eyes. When we added food color in milk, the molecules of food colors also move with fat molecules and we can see the chemical reaction through the movement of food color.
The entire process is very fast and it displays as scattering of food colors almost as beautiful explosion of colors.
- Subscribe Sameer Goyal at ekunji to get easy science projects and craft ideas for kids.
- Subscribe Sameer Goyal at Youtube channel for more science projects and craft ideas videos.
- Join Sameer Goyal at Facebook
- Join Sameer Goyal at Pinterest
- Join Sameer Goyal at Twitter
- Join Sameer Goyal at Google+
Try this easy science experiment to demonstrate your kids that how soap works and write me your feedback in comment area below.

