Conductivity of water is one of the very common science experiments for kids in their science syllabus. Kids read about electricity, conductivity and electrolysis of water and brine solution in their books. These processes are explained theoretically in their books and they always want to do it themselves.
Today, I’m presenting here this science experiment for kids in a very easy and simple way with general household items so that your kids can try this conductivity of water experiment at home by their own under your supervision.
After all, these science experiments for kids are the best way to learn with fun. Isn’t it?
Your kids can also use this experiment for their science fair projects.
Apparatus Required For Conductivity of Water Experiment :
- Drinking Glass – 1
- Metal clamps – 4
- Pieces of electrical wire – 3
- LED bulb – 1 (you can get it from any fancy LED light, i.e. Christmas lights)
- Small battery – 1 (you can find it in toys and many general household items)
- Distilled water – 1 glass
- Salt – 2 tea spoon
How To Setup Circuit for Conductivity of Water Experiment :
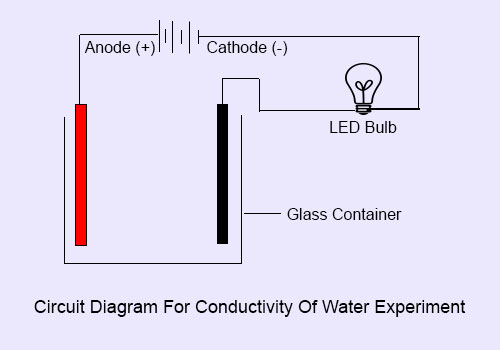
1. First of all attach 2 metal clamps to both the ends of one wire.
2. Then attach one clamp to one end of 2nd wire and attach one clamp to one end of 3rd wire. Leave one end of both the wires free.
3. Join LED bulb with free ends of 2nd and 3rd wire.
4. Take 1st wire which have clamps at both the ends and fix it’s one clamp with the glass rim and fix another clamp to one terminal of battery.
5. Now take another wire which is attached with LED bulb and fix it’s one clamp on glass rim and fix another clamp to the second terminal of battery.
6. Setup of circuit for the experiment is complete.
Conductivity of Water Experiment : Video
Steps For Conductivity of Water Experiment :
Experiment 1 :-
1. Fill distilled water in glass up to the rim so that, the clamps get half immersed in water.
2. You will notice that the bulb doesn’t glow.
Result of Experiment 1 :-
It means, the distilled water is bad conductor of electricity.
Experiment 2 :-
1. Now the time for magic. Add 2 teaspoon salt in water and mix properly.
2. See the bulb glows instantly as you dissolved the salt in water.
Result of Experiment 2 :-
It means brine solution is good conductor of electricity.
Do you know why it happens?
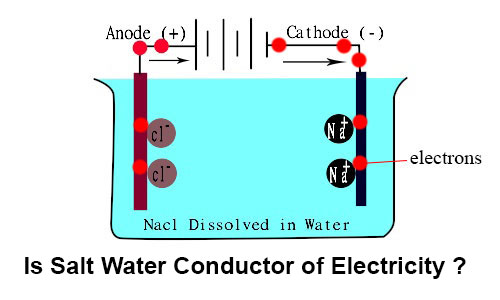
When electricity passed from brine solution then salt (Nacl) gets ionized and 2 types of ions are formed :-
- Positive ions of Sodium (Na+)
- Negative ions of Chlorine (Cl-)
Sodium (Na+) ions always flow towards the negative electrode (cathode). Negative chlorine (Cl-) ions always flow towards the positive electrode (Anode).
When Na+ ions collide with cathode, these ions pick up electrons. On the other hand, when Cl- ions collide with anode these ions release electrons.
This way the flow of electrons starts in brine solution with the help of salt. Thus the electrical circuit completes and the bulb glows.
Whereas, in distilled water, there was no carrier for electrons between two electrodes. Therefore the circuit did not completed and electricity didn’t flow in distilled water.
- Subscribe Sameer Goyal at ekunji to get easy science projects and craft ideas for kids.
- Subscribe Sameer Goyal at Youtube channel for more science projects and craft ideas videos.
- Join Sameer Goyal at Facebook
- Join Sameer Goyal at Pinterest
- Join Sameer Goyal at Twitter
- Join Sameer Goyal at Google+
Try this easy science experiment to demonstrate conductivity of water and brine solution and write me your feedback in comment area below.

Pingback: Water Conductivity Science Experiment - पानी की सुचालकता का प्रयोग